Modelling Rhenium-SCT® Dose Distributions Using Monte Carlo Methods: A Foundational Dosimetry Study
Background and Rationale
In the radiation oncology setting, clinicians and medical physicists are familiar with the fundamentals of radiation therapy and dosimetry; particularly regarding the interaction of X-rays, electrons, and radioisotope sources within human tissue, as seen in treatments like external beam radiation therapy and brachytherapy.
Rhenium-SCT® is an innovative treatment that utilises a resin matrix bonded with the beta emitter radioisotope, rhenium-188, to treat Non-Melanoma Skin Cancer (NMSC). Rhenium-SCT® is designed to deliver high-dose, conformal radiation to the lesion surface with rapid dose fall-off, making it particularly suited for complex-shaped, shallow lesions located in cosmetically or functionally sensitive areas such as the nose, ears, or perioral region. The integration of Rhenium-SCT® into radiotherapy clinics provides a unique opportunity to develop verification and validation standards for beta radiation delivery, specifically by visualising and quantifying the emission profile of rhenium-188 in tissue. This enables contextualisation of Rhenium-SCT® within the broader radiation therapy landscape and supports its safe and effective application alongside established techniques.
The development of 3D dose distributions for rhenium-188 will support improved visualisation and facilitate more meaningful comparisons with conventional EBRT modalities.
To address these foundational questions, OncoBeta Therapeutics commissioned an independent physicist, in collaboration with the University of Wollongong, to model dose deposition using the Monte Carlo toolkit, Geant4. Geant4 has been extensively validated for a variety of medical physics applications1, demonstrating confidence in the results it produces. Through utilising this tool, this study intended to investigate the physical properties of rhenium-188 and its clinical value for patients with non-melanoma skin cancer.
Research Project Aims
The research project focused on achieving three major goals:
- Monte Carlo Modelling of Dose Deposition
- Model the dose distribution of rhenium-188 in a water phantom, a current industry standard for simulating human tissue in radiotherapy research.
- Using Geant4 Monte Carlo methods, model beta particle movement and interaction from rhenium-188, to produce data that could be directly compared to conventional methods, including electron beams and superficial X-rays
- Benchmark the simulated rhenium-188 dose profile against a 4 MeV electron beam due to similar clinical dose deposition characteristics.
- Evaluate Lateral Dose Falloff Assessment
- Examine radiation dose distribution behaviour at the lateral boundaries of the treatment field which forms the ‘penumbra’ in external beam therapy. This is a necessary consideration when determining treatment margins and avoiding unnecessary exposure to adjacent healthy tissues.
- Quantify the falloff at the treatment boundaries and provide clinicians with familiar metrics to aid comparison. A steeper dose gradient at the edge indicates more precise targeting, which is particularly beneficial in the treatment of superficial skin cancers.
- Determine the Impact of Paste Layer Thickness
- Investigate the impact of applying multiple or excessively thick layers of the resin. Unlike other radiotherapy techniques, a portion of the rhenium-188 dose is absorbed within the paste itself, with the extent of absorption varying by thickness. The hypothesis was that overly thick layers do not meaningfully increase dose at depth and may be inefficient.
- Model the degree of dose absorption within the resin and calculate the variability introduced by differences in application thickness. This information is essentially useful for guiding clinicians on optimal resin application; ensuring effective treatment while avoiding under-dosing, over-dosing, or unnecessary resin waste.
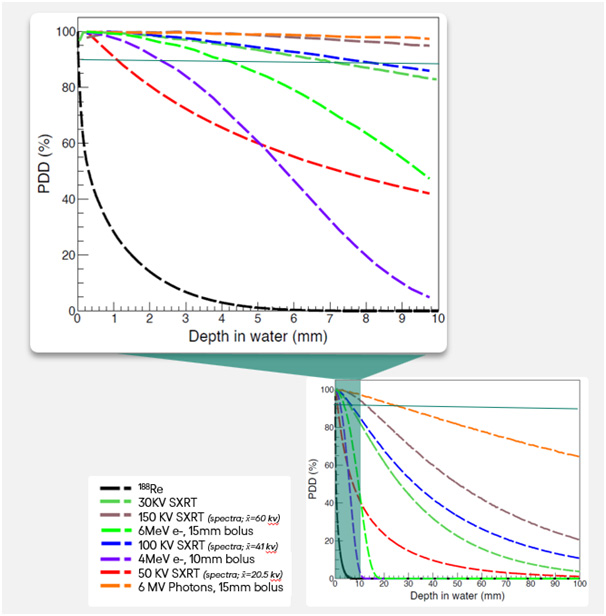
Percentage Depth Dose (PDD) and Rhenium-188
In radiation oncology, dosimetric analysis depends fundamentally upon PDD curves. In traditional external beam radiation oncology, these graphs illustrate how the absorbed dose changes with depth in water, providing a visual reference for the penetration and distribution of radiation beams.
The study presented a PDD-equivalent curve for rhenium-188 superimposed on typical curves for superficial X-rays and various electron beams, including near analogous modalities like 4MeV. Data analysis revealed a steep dose reduction for rhenium-188 which demonstrates precise and potent surface targeting while keeping deeper tissue exposure low.
Compared to electrons and photons, rhenium-188 not only limits unnecessary deep tissue irradiation but also maintains strong tumour control at the surface. These features demonstrate that Rhenium-SCT® fulfils its intended use case as a highly localised, tissue-sparing treatment option.
| Modality | Clinically Effective Depth* | Clinical Applications | Pros | Cons |
| 4 MeV electrons + bolus | ≤2.5 mm2,3 | Small BCCs, extremities | Compared to photons, dose falloff spares deep tissues4 Ideal for 3-25 mm lesions2 | Requires bolus for optimal surface dose2 Field size limitations2 Not ideal for non-flat surfaces, e.g. the nasal bridge and conchal bowl. Penumbra margin increases surface area treated |
| 6 MeV electrons + bolus | ≤5 mm4,5 | Thicker SCC/BCC, keloids | ||
| 30 kV SXRT | ≤3 mm6 | Actinic keratosis; Bowen’s disease; superficial BCC/SCC | Precise superficial targeting (0.5–15 mm)6 No scarring Suitable for frail patients or non-surgical candidates Patient does not have to lie flat; can be treated sitting position | Limited to shallow depths Requires 5–15 sessions Higher recurrence risk for thick tumours10 |
| 50 kV SXRT† | ≤5 mm7,8 | NMSC up to 5 mm depth; keloids; palliative bony metastases (e.g., ribs) | ||
| 100 kV SXRT† | ≤9 mm6 | NMSC up to 9 mm depth; large lesions on face/neck; recurrent tumours after surgery | ||
| 150 kV SXRT† (Orthovoltage) | ≤15 mm6 | Deep dermal SCC/BCC; palliative bone pain (e.g., sternum) | ||
| 6 MV photons + bolus | ≤30 mm (single beam)9 | Advanced/recurrent tumours invading muscle; palliative bulky lesions (e.g., scalp); post-surgical prophylaxis | Treats deeper tumours (>30 mm)9Widely available due to Linac use6 | Poor skin dose coverage (skin-sparing effect)11 Risks to bone/organs7Higher integral dose11 |
| Rhenium-SCT® | ≤3 mm12 | Early-stage BCC and SCC, including recurrences; Lesions < 3mm deep | Single session treatmentIdeal for complex surfaces Avoids OARs | Patient batching required Shallow lesions |
– Depth receiving ≥90% of Dmax
† Energies on the PDD graph represent spectra calculations rather than monoenergetic values
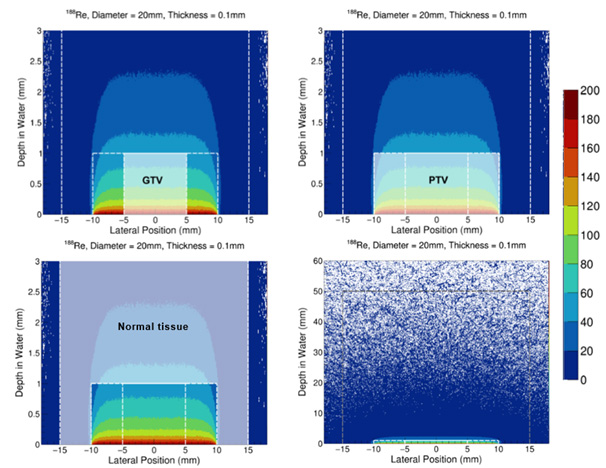
Simulated Tumour and Gross and Clinical Treatment Volumes (GTV/CTV)
To enable meaningful comparison, a simulated treatment plan was developed for a representative tumour volume and corresponding clinical margins and then evaluated using both rhenium-188 and a 4 MeV electron beam. The dose was prescribed to deliver 50 Gy at a depth of 1 mm for both modalities.
To conduct the dosimetric analysis, a tumour structure was generated with an additional clinical treatment margin, with a larger external structure serving as the ‘normal tissue’ for toxicity measurements. This approach mirrored conventional electron therapy planning methods, using standardised dosimetric principles to ensure a fair and clinically relevant comparison. By aligning the evaluation framework with established practices, the results are more easily interpretable and translatable to routine clinical decision-making.
The size and shape of the simulated structures were derived from standardised dimensions based on previously treated Rhenium-SCT® cases and aligned with current patient selection criteria for this therapy.
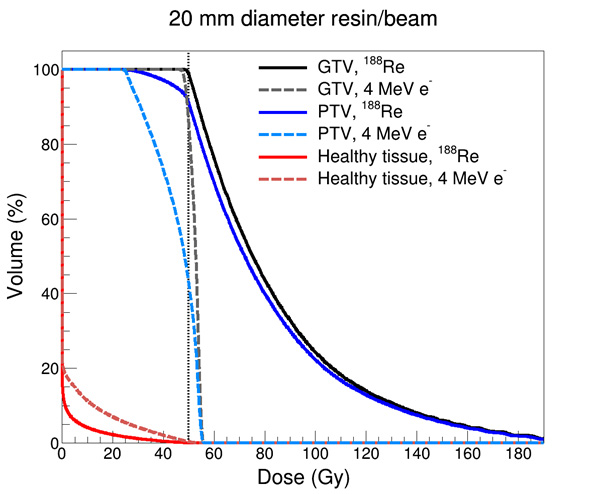
Dose Volume Histogram (DVH) Comparison
DVH graphs are typically used to illustrate the distribution of radiation dose received by both tumour and adjacent healthy tissues. Based on the simulated treatment volumes, DVH analysis in this study showed that rhenium-188 provides superior sparing of healthy tissues compared to a 4 MeV electron beam, while still achieving comparable or enhanced coverage of target volumes. A 4 MeV electron beam was selected as an appropriate comparator as other modalities, such as SXRT, are expected to offer less favourable tissue-sparing characteristics based on PDD curves for these modalities.
This outcome is attributed to the steeper dose gradient and reduced lateral scatter associated with rhenium-188. Notably, in superficial tumour regions, the rhenium-188 model demonstrated a relative dose increase on the skin surface due to the application mechanism of the resin ³. It is of note however the that while this surface dose appears excessive graphically, the total volume of the treatment area was only 0.314 cm3, indicating that only a very small proportion of skin is receive such high doses, and this is within the gross tumour region.
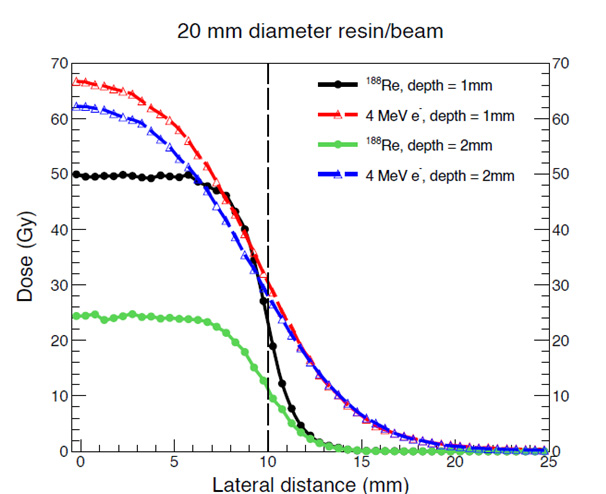
Lateral Profile Modelling
Comparative analysis of lateral dose profiles further supported the advantages of rhenium-188 in its application. Its profile exhibited a more uniform and predictable dose distribution at depth, whereas the electron beam showed increased curvature and variability.
At the field margins, rhenium-188 demonstrated a sharper dose fall-off, minimising radiation to surrounding non-target tissues while maximising the tumour volume coverage of curative dose. This steep gradient enhances the therapeutic ratio and reduces the likelihood of adverse effects in adjacent healthy skin.
The Impact of Resin Thickness
A unique consideration for Rhenium-SCT® is the influence of resin thickness on dose delivery. Because rhenium-188 emits beta radiation, the dose is partially attenuated by the resin before reaching the target tissue.
Modelling indicated that resin thicknesses of 0.15 mm and 0.3 mm result in approximately 2% and 5% dose absorption, respectively. While the standard resin thickness used in clinical practice is 0.1 mm, thicker applications would require small adjustments to treatment times to compensate for attenuation. Resin thicknesses above 0.3 mm are generally not considered clinically practical due to operational nuances in the nature of the resin application process.
Despite these variations, clinical outcomes have demonstrated consistently high tumour control rates and a favourable safety profile across a wide range of NMSC subtypes and histologies, anatomical locations, and tumour characteristics. This suggests that when delivering a therapeutic dose of 50 Gy at a specified depth, minor variations in resin thickness do not significantly compromise clinical efficacy13.
Conclusion
This modelling study provides insight into the physical behaviour and clinical potential of rhenium-188 for the treatment of NMSC. Using Geant4 Monte Carlo simulations, the project successfully addressed its aims in modelled rhenium-188 dose deposition in a water phantom, benchmarked lateral dose falloff against a 4 MeV electron beam, and quantified the effect of varying resin thickness on dose attenuation and delivery.
Across all objectives, Rhenium-SCT® demonstrated key clinical advantages. It produced a highly conformal PDD profile, with steep dose gradients that enhance surface targeting while sparing deeper tissues. Compared to a 4 MeV electron beam, rhenium-188 achieved slightly uniform tumour coverage with a sharper lateral falloff and dose drop-off at depth, resulting in improved sparing of adjacent healthy skin. Furthermore, modelling confirmed that typical variations in resin thickness had only minimal yet easily measurable and manageable impact on delivered dose, supporting the robustness of clinical application techniques.
Ultimately, these findings support the clinical evidence generated to date that Rhenium-SCT® is a precise, surface-conformal radiotherapeutic option, which is suited to irregular anatomical regions and patients unsuitable for surgery or fractionated radiotherapy.
Want to learn more about this report?
References:
- Arce P, Archer JW, Arsini L, Bagulya A, Bolst D, Brown JMC, et al. Results of a Geant4 benchmarking study for bio-medical applications, performed with the G4-Med system. Med Phys. 2025 May;52(5):2707-2761. doi: 10.1002/mp.17678. Epub 2025 Feb 21. PMID: 39981742; PMCID: PMC12059550.
- Valve A, Koskenmies S, Tenhunen M, Nurmi H, Hernberg M, Salminen S, Anttonen A. Early clinical experience with a degraded 4 MeV electron beam in radiotherapy of superficial basal cell carcinoma. Phys Imaging Radiat Oncol. 2023 Aug 28;27:100487. doi: 10.1016/j.phro.2023.100487. PMID: 37705728; PMCID: PMC10495663.
- Gerbi BJ, Antolak JA, Deibel FC, Followill DS, Herman MG, Higgins PD, et al. Recommendations for clinical electron beam dosimetry: Supplement to the recommendations of Task Group 25. Med Phys. 2009 Jun;36(7):3239-79. doi: 10.1118/1.3125820.
- Top 3 pros and cons of electron beam therapy. Ebeam Machine [Internet]. 2024 Dec 3 [cited 2025 May 22]. Available from: https://ebeammachine.com/top-3-pros-and-cons-of-electron-beam-therapy/
- OncoLink. Electron beam radiation therapy. OncoLink [Internet]. [cited 2025 May 23]. Available from: https://www.oncolink.org/cancer-treatment/radiation/types-of-radiation-therapy/electron-beam-radiation-therapy
- Radiation Oncology Targeting Cancer. Essential radiotherapy techniques – superficial and orthovoltage. Radiation Oncology Targeting Cancer [Internet]. [cited 2025 May 23]. Available from: http://www.radiationoncology.com.au/resourcing-the-radiation-oncology-sector/essential-radiotherapy-techniques-superficial-and-orthovoltage/
- Sheu RD, Powers A, Lo YC. Commissioning a 50-100 kV X-ray unit for skin cancer treatment. J Appl Clin Med Phys. 2015 Mar 8;16(2):5182. doi: 10.1120/jacmp.v16i2.5182. PMID: 26103186; PMCID: PMC5690081.
- Yu L, Oh C, Shea CR. The Treatment of Non-Melanoma Skin Cancer with Image-Guided Superficial Radiation Therapy: An Analysis of 2917 Invasive and In Situ Keratinocytic Carcinoma Lesions. Oncol Ther. 2021 Jun;9(1):153-166. doi: 10.1007/s40487-021-00138-4. Epub 2021 Feb 5. PMID: 33547631; PMCID: PMC8140015.
- Sen S, Bandyopadhyay A, Pal JK, Ghosh AK, Deb AR. A dosimetric study of electron beam therapy vs. high-dose-rate mould brachytherapy in adjuvant treatment of non-melanoma skin carcinomas of the head and neck region. J Contemp Brachytherapy. 2019 Dec;11(6):547-553. doi: 10.5114/jcb.2019.90233. Epub 2019 Dec 1. PMID: 31969913; PMCID: PMC6964343.
- American Cancer Society. Radiation therapy for basal and squamous cell skin cancers. American Cancer Society [Internet]. [cited 2025 May 23]. Available from: https://www.cancer.org/cancer/types/basal-and-squamous-cell-skin-cancer/treating/radiation-therapy.html
- Yosefof E, Kurman N, Yaniv D. The Role of Radiation Therapy in the Treatment of Non-Melanoma Skin Cancer. Cancers (Basel). 2023 Apr 22;15(9):2408. doi: 10.3390/cancers15092408. PMID: 37173875; PMCID: PMC10177122.
- Tietze JK, Heuschkel M, Krönert MIC, Kurth J, Bandow G, Ojak G, et al. Topical 188Re ionizing radiation therapy exerts high efficacy in curing nonmelanoma skin cancer. Clin Nucl Med. 2023 Oct;48(10):869-876. doi: 10.1097/RLU.0000000000004824.
- Baxi S, Vohra S, Hong A, Mulholland N, Heuschkel M, Dahlhoff G, et al. Effectiveness and patient experiences of rhenium skin cancer therapy for nonmelanoma skin cancer: interim results from the EPIC-Skin Study. J Nucl Med. 2024 Jul [cited 2025 May 23]. doi: 10.2967/jnumed.124.267988